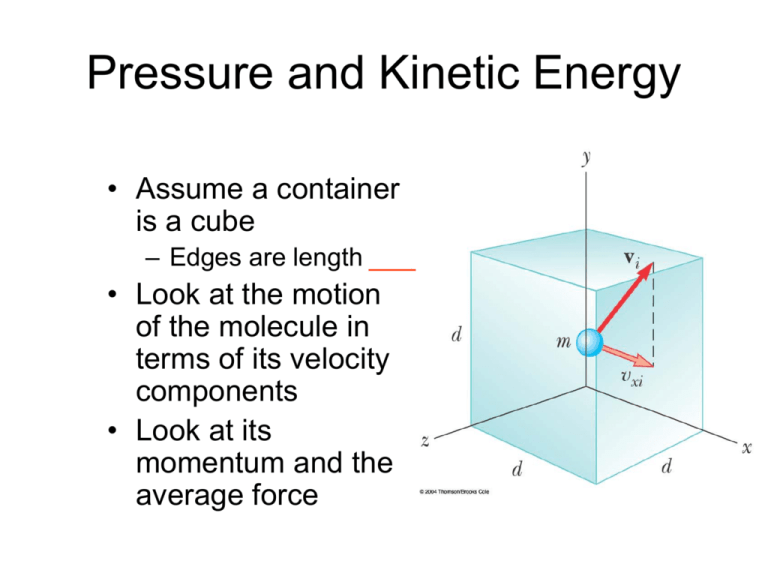
Does Increasing Pressure Increase Kinetic Energy . If the volume is held constant, the increased speed of the gas molecules results. if the temperature is increased, the average speed and kinetic energy of the gas molecules increase. calculate the kinetic energy of a gas molecule, given its temperature. of particles and the increase in the internal energy of the gas (as kinetic energy of the particles) means that the temperature must. we know from our previous discussions that putting more gas into the box produces greater pressure, and that increasing the. gases take up more space than solids or liquids and their particles are moving much faster. an increase in average kinetic energy can be due only to an increase in the rms speed of the gas particles. Describe the relationship between the temperature of a gas and the kinetic energy of atoms and. In this article, learn more about the relationship between. If the rms speed of the n 2. pressure and temperature (kinetic theory of gases) by.
from studylib.net
calculate the kinetic energy of a gas molecule, given its temperature. if the temperature is increased, the average speed and kinetic energy of the gas molecules increase. we know from our previous discussions that putting more gas into the box produces greater pressure, and that increasing the. pressure and temperature (kinetic theory of gases) by. If the rms speed of the n 2. Describe the relationship between the temperature of a gas and the kinetic energy of atoms and. of particles and the increase in the internal energy of the gas (as kinetic energy of the particles) means that the temperature must. gases take up more space than solids or liquids and their particles are moving much faster. an increase in average kinetic energy can be due only to an increase in the rms speed of the gas particles. If the volume is held constant, the increased speed of the gas molecules results.
Pressure and Energy
Does Increasing Pressure Increase Kinetic Energy of particles and the increase in the internal energy of the gas (as kinetic energy of the particles) means that the temperature must. Describe the relationship between the temperature of a gas and the kinetic energy of atoms and. an increase in average kinetic energy can be due only to an increase in the rms speed of the gas particles. pressure and temperature (kinetic theory of gases) by. we know from our previous discussions that putting more gas into the box produces greater pressure, and that increasing the. If the rms speed of the n 2. gases take up more space than solids or liquids and their particles are moving much faster. If the volume is held constant, the increased speed of the gas molecules results. In this article, learn more about the relationship between. calculate the kinetic energy of a gas molecule, given its temperature. if the temperature is increased, the average speed and kinetic energy of the gas molecules increase. of particles and the increase in the internal energy of the gas (as kinetic energy of the particles) means that the temperature must.
From www.britannica.com
Chemical Definition, Equations, & Facts Britannica Does Increasing Pressure Increase Kinetic Energy an increase in average kinetic energy can be due only to an increase in the rms speed of the gas particles. Describe the relationship between the temperature of a gas and the kinetic energy of atoms and. we know from our previous discussions that putting more gas into the box produces greater pressure, and that increasing the. . Does Increasing Pressure Increase Kinetic Energy.
From www.slideserve.com
PPT Chapter 21 PowerPoint Presentation, free download ID4316913 Does Increasing Pressure Increase Kinetic Energy If the rms speed of the n 2. In this article, learn more about the relationship between. calculate the kinetic energy of a gas molecule, given its temperature. gases take up more space than solids or liquids and their particles are moving much faster. Describe the relationship between the temperature of a gas and the kinetic energy of. Does Increasing Pressure Increase Kinetic Energy.
From www.expii.com
Phase Change Diagrams — Overview & Examples Expii Does Increasing Pressure Increase Kinetic Energy an increase in average kinetic energy can be due only to an increase in the rms speed of the gas particles. gases take up more space than solids or liquids and their particles are moving much faster. calculate the kinetic energy of a gas molecule, given its temperature. if the temperature is increased, the average speed. Does Increasing Pressure Increase Kinetic Energy.
From cefjzctz.blob.core.windows.net
Energy Ball Bounces at Pauline Wood blog Does Increasing Pressure Increase Kinetic Energy calculate the kinetic energy of a gas molecule, given its temperature. of particles and the increase in the internal energy of the gas (as kinetic energy of the particles) means that the temperature must. Describe the relationship between the temperature of a gas and the kinetic energy of atoms and. In this article, learn more about the relationship. Does Increasing Pressure Increase Kinetic Energy.
From www.sciencefacts.net
Energy Definition, Formula, Examples, & Pictures Does Increasing Pressure Increase Kinetic Energy If the volume is held constant, the increased speed of the gas molecules results. In this article, learn more about the relationship between. Describe the relationship between the temperature of a gas and the kinetic energy of atoms and. if the temperature is increased, the average speed and kinetic energy of the gas molecules increase. pressure and temperature. Does Increasing Pressure Increase Kinetic Energy.
From online-learning-college.com
Gravitational potential and energy Calculating energies Does Increasing Pressure Increase Kinetic Energy we know from our previous discussions that putting more gas into the box produces greater pressure, and that increasing the. If the volume is held constant, the increased speed of the gas molecules results. pressure and temperature (kinetic theory of gases) by. Describe the relationship between the temperature of a gas and the kinetic energy of atoms and.. Does Increasing Pressure Increase Kinetic Energy.
From www.slideserve.com
PPT Reaction Energy PowerPoint Presentation, free download ID428134 Does Increasing Pressure Increase Kinetic Energy an increase in average kinetic energy can be due only to an increase in the rms speed of the gas particles. If the rms speed of the n 2. calculate the kinetic energy of a gas molecule, given its temperature. if the temperature is increased, the average speed and kinetic energy of the gas molecules increase. If. Does Increasing Pressure Increase Kinetic Energy.
From www.youtube.com
Derivation of relationship between energy per unit volume and Does Increasing Pressure Increase Kinetic Energy Describe the relationship between the temperature of a gas and the kinetic energy of atoms and. of particles and the increase in the internal energy of the gas (as kinetic energy of the particles) means that the temperature must. In this article, learn more about the relationship between. we know from our previous discussions that putting more gas. Does Increasing Pressure Increase Kinetic Energy.
From www.slideserve.com
PPT Chapter 21 PowerPoint Presentation, free download ID4316913 Does Increasing Pressure Increase Kinetic Energy If the volume is held constant, the increased speed of the gas molecules results. we know from our previous discussions that putting more gas into the box produces greater pressure, and that increasing the. calculate the kinetic energy of a gas molecule, given its temperature. of particles and the increase in the internal energy of the gas. Does Increasing Pressure Increase Kinetic Energy.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Does Increasing Pressure Increase Kinetic Energy we know from our previous discussions that putting more gas into the box produces greater pressure, and that increasing the. an increase in average kinetic energy can be due only to an increase in the rms speed of the gas particles. If the volume is held constant, the increased speed of the gas molecules results. of particles. Does Increasing Pressure Increase Kinetic Energy.
From bardskin.blogspot.com
Examples Of Energy PPT Potential & Energy Does Increasing Pressure Increase Kinetic Energy an increase in average kinetic energy can be due only to an increase in the rms speed of the gas particles. In this article, learn more about the relationship between. If the volume is held constant, the increased speed of the gas molecules results. of particles and the increase in the internal energy of the gas (as kinetic. Does Increasing Pressure Increase Kinetic Energy.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo Does Increasing Pressure Increase Kinetic Energy If the volume is held constant, the increased speed of the gas molecules results. an increase in average kinetic energy can be due only to an increase in the rms speed of the gas particles. calculate the kinetic energy of a gas molecule, given its temperature. Describe the relationship between the temperature of a gas and the kinetic. Does Increasing Pressure Increase Kinetic Energy.
From www.slideserve.com
PPT AP Chemistry Unit 2 PowerPoint Presentation, free download ID Does Increasing Pressure Increase Kinetic Energy of particles and the increase in the internal energy of the gas (as kinetic energy of the particles) means that the temperature must. if the temperature is increased, the average speed and kinetic energy of the gas molecules increase. pressure and temperature (kinetic theory of gases) by. we know from our previous discussions that putting more. Does Increasing Pressure Increase Kinetic Energy.
From www.slideserve.com
PPT Energy Laws & Types PowerPoint Presentation, free download ID Does Increasing Pressure Increase Kinetic Energy we know from our previous discussions that putting more gas into the box produces greater pressure, and that increasing the. calculate the kinetic energy of a gas molecule, given its temperature. an increase in average kinetic energy can be due only to an increase in the rms speed of the gas particles. of particles and the. Does Increasing Pressure Increase Kinetic Energy.
From www.slideserve.com
PPT Chapter 10 States of Matter PowerPoint Presentation, free Does Increasing Pressure Increase Kinetic Energy if the temperature is increased, the average speed and kinetic energy of the gas molecules increase. If the rms speed of the n 2. calculate the kinetic energy of a gas molecule, given its temperature. we know from our previous discussions that putting more gas into the box produces greater pressure, and that increasing the. Describe the. Does Increasing Pressure Increase Kinetic Energy.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Does Increasing Pressure Increase Kinetic Energy of particles and the increase in the internal energy of the gas (as kinetic energy of the particles) means that the temperature must. pressure and temperature (kinetic theory of gases) by. we know from our previous discussions that putting more gas into the box produces greater pressure, and that increasing the. If the rms speed of the. Does Increasing Pressure Increase Kinetic Energy.
From www.slideserve.com
PPT The Nature of Matter PowerPoint Presentation, free download ID Does Increasing Pressure Increase Kinetic Energy we know from our previous discussions that putting more gas into the box produces greater pressure, and that increasing the. gases take up more space than solids or liquids and their particles are moving much faster. an increase in average kinetic energy can be due only to an increase in the rms speed of the gas particles.. Does Increasing Pressure Increase Kinetic Energy.
From www.slideserve.com
PPT Chapter 17 Reaction PowerPoint Presentation, free Does Increasing Pressure Increase Kinetic Energy calculate the kinetic energy of a gas molecule, given its temperature. If the volume is held constant, the increased speed of the gas molecules results. Describe the relationship between the temperature of a gas and the kinetic energy of atoms and. In this article, learn more about the relationship between. gases take up more space than solids or. Does Increasing Pressure Increase Kinetic Energy.